LAST APRIL, A GROUP OF RESEARCHERS FROM THE UNIVERSITY OF ALBERTA (CANADA) PUBLISHED AN INTERESTING IN VITRO STUDY ON THE CYTOPROTECTIVE ACTIVITY OF EXTRACELLULAR VESICLES (EVs) EXPRESSING AMYLIN RECEPTOR (AMY1-3) AGAINST THE TOXICITY OF ßAMYLOID PROTEIN.
Amylin is a hormone produced by pancreatic cells, capable of forming accumulations similar to those characterizing Alzheimer’s disease. The amylin receptor belongs to a class of B G-protein-coupled receptors and is a heterodimer composed by the calcitonin receptor and the receptor modifying the activity of proteins.
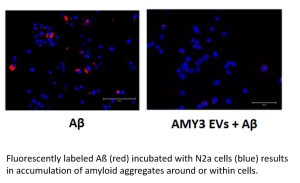
After isolation from embryonic human kidney cells, EVs expressing the AMY1-3 receptor were characterized by Western Blot, Electron Microscopy and Dynamic Light Scattering (DLS) techniques in order to determine the diameter and the presence of specific proteins. Then EVs were incubated for 48h with cells of mouse neuroblastoma (N2a) in presence of soluble ßamyloid oligomers.
IT WAS OBSERVED A REDUCTION IN THE CYTOTOXICITY OF ßAMYLOID AND CONSEQUENTLY A GREATER SURVIVAL OF N2a CELLS IN THE PRESENCE OF EVs EXPRESSING THE AMY3 RECEPTOR SUBTYPE, THANKS TO A STRONG BINDING AFFINITY BETWEEN THE RECEPTOR ITSELF AND ßAMYLOID OLIGOMERS.
Further confirmation was obtained by pre-treating the AMY3 EVs with an amylin antagonist ligand and subsequently incubating them as previously described. In this case, a slight decrease in the cytoprotective capacity of the EVs was observed.
Another important result, obtained by the researchers, was observed after Long Term Potentiation (LTP) induction in mouse hippocampal brain slices and in presence of ßamyloid. EVs expressing the amylin receptor and EVs not expressing this receptor, were incubated. However, an improvement in Long Term Potentiation was observed only after the perfusion of EVs expressing AMY3 receptor. This was confirmed by using in vivo mouse model with a genetic depletion of calcitonin receptors, indeed EVs from their brain demonstrated less binding to ßamyloid oligomers than a mice without genetic mutation.
THESE RESULTS ARE TO BE CONSIDERED PROMISING, ESPECIALLY BEACAUSE THEY COULD REPRESENT A VALID AND NEW THERAPEUTIC TARGET FOR ALZHEIMER’S DISEASE!
Here the link: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0267164