LABION POSITION IN THE EUROPEAN POLICY MAKING IN NANOMEDICINE
LABION is highly involved in the international effort in nanomedicine, both at the scientific and at the policy-making levels. The main channel for this involvement is the European Technology Platform in Nanomedicine (ETPN), in which Fondazione Don Gnocchi was one of the co-founders in 2005. LABION has participated in several EU funded projects, on behalf of Fondazione don Gnocchi, in Framework Programme 7, including the Photonics4Life Network of Excellence.
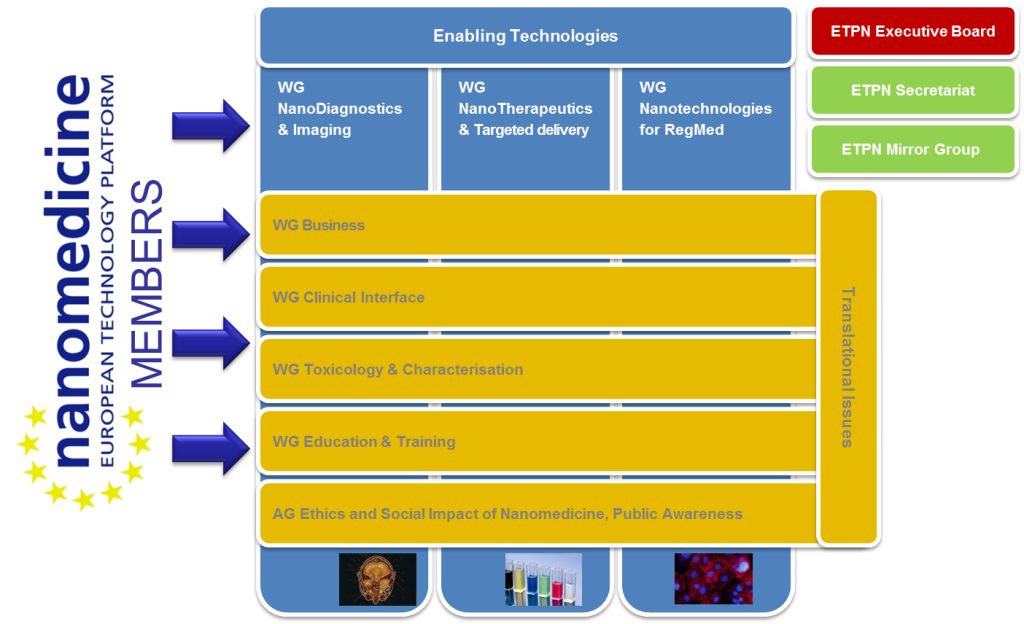
The European Technology Platform Nanomedicine was established in 2005 as a joint venture of the European Commission and CEOs of large industrial companies, SMEs and academic research institutions to investigate and advance joint activities in the area of nanotechnology in medicine. Since 2005 the ETPN published a number of strategic documents outlining the needs and roadmaps for nanomedicine research in Europe. The ETPN contributed to set up numerous European funded projects providing a first impression of the conditions for a suitable social and economic environment and the structural requirements for an efficient translation of R&D results into innovative nanomedicine. The ETPN supports its members in coordinating their joint research efforts and improving communication amongst the members as well as towards the European Commission and the European Member States.
The association gathers today more than 125 members from 25 different Member States, covering all stakeholders of Nanomedicine : academia, SMEs, industry, pubic agencies, representatives from national platforms, European Commission, etc.
The strategic research priorities of the ETP Nanomedicine represent the core fields of interest and activities of the members of the technology platform: Regenerative Medicine and Biomaterials, Nanotherapeutics (including drug delivery), Medical devices including Nanodiagnostics and Imaging.
For more information, visit www.etp-nanomedicine.eu.
Nano-concepts for a macro-impact on EU economy: the ETPN White Paper on Contribution of Nanomedicine to Horizon 2020 and the EU-Nanomedicine Map
The ETPN White Paper is a strategic document that provides a set of key recommendations for the European Commission and the EU Member States to create a favorable ecosystem for the successful deployment of Nanomedicine in Europe. It lays thereby the groundwork to manage the efficient translation of nanotechnology from a Key Enabling Technology (KET) into new and innovative medical products.
The ETPN White Paper recommendation has been thoroughly taken into account by European Commission to set-up nanomedicine-related aspects of Work Programmes in Horizon 2020.
In summary, the pivotal proposition of the White Paper released in 2013 is the establishment of a Nanomedicine Translation Hub designed as an umbrella for a set of complementary actions and initiatives such as:
- a dedicated Nanomedicine Translation Advisory Board (TAB) with experienced industrial experts to select, guide and push forward the best translatable concepts,
- a European Nano-Characterisation Laboratory (EU-NCL) for physical, chemical and biological characterisation of nanomaterials intended for medical use,
- GMP manufacturing pilot lines for clinical batches to assist both academia and SMEs to develop nanomedical materials for validation in clinical trials, before transfer to dedicated manufacturing organisations, a dedicated NanoMed SME instrument aiming at funding discovery projects and innovative SMEs in order to keep excellence in nanomedicine research and more importantly develop products.
The White Paper “Contribution of Nanomedicine to Horizon 2020” is available for download on the ETP Nanomedicine website under www.etp-nanomedicine.eu/etpn-white-paper-2013
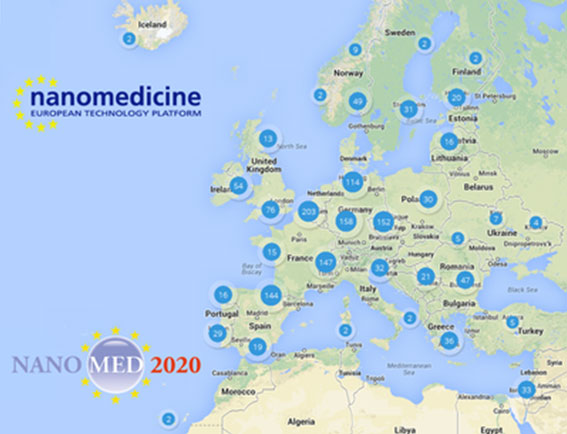
Another result of the effort of the ETPN (and of its members) is the Map of the Nanomedicine stakeholders in Europe. The map gives a very useful information in term of understanding “who does what” and “who is where” in nanomedicine, this new tool identifies actors with peculiar knowledge and/or infrastructure which can fill a gap or optimize processes, ideas and projects.
Explore the – not so small – European nanomedicine community on the ETPN website at: http://www.etp-nanomedicine.eu/public/european-nanomedicine-map